网站维护
系统内容更新/升级中

2020/12/21

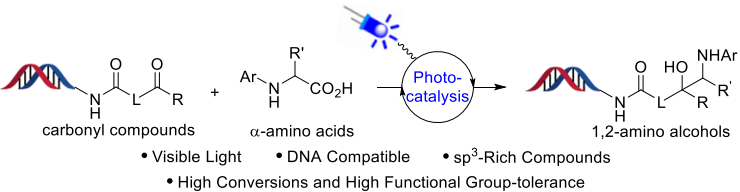
DNA-encoded library (DEL) technology is a powerful and valuable tool commonly used by the pharmaceutical industry for the identification of compounds with affinity to biomolecular targets. Success in this endeavor lies in sampling diverse chemical libraries. However, current DNA-encoded library technology tend to be deficient in C(sp3) carbon counts.
Recently, WuXi AppTec HitS Unit has reported a DNA-compatible photoredox decarboxylative coupling reaction between α-amino acids and carbonyl compounds for the efficient synthesis of DNA-conjugated sp3-rich 1,2-amino alcohols. The reaction proceeds efficiently for a wide range of DNA-conjugated aldehydes and ketones under mild conditions, and provides the desired 1,2-amino alcohols with high conversions, enjoys huge potential and broad prospect in the DNA-encoded library production. Also, qPCR and sequencing data analysis indicates no significant DNA damage upon photoredox decarboxylative coupling reaction.
This work has been published on Organic Letters as the cover article.
Reference:
Wen, Huanan; Ge, Rui; Qu, Yi; Sun, Jialin; Shi, Xiaodong; Cui, Weiren; Yan, Hao; Zhang, Qi; An, Yulong; Su, Wenji; Yang, Hongfang; Kuai, Letian; Satz, Alexander. L.; Peng, Xuanjia. Org. Lett. 2020, 22(24), 9484-9489. DOI:10.1021/acs.orglett.0c03461