网站维护
系统内容更新/升级中

Undesirable off-target activity profiles could hinder or halt the development of candidate drugs or even lead to market withdrawal if discovered after a drug is approved. To tackle this issue early in drug development continuum, in vitro pharmacological profiling has been widely used by pharmaceutical companies to identify off-target effects. This low cost “early” strategy has proved to help identify potential ADRs (Adverse drug reactions) and increase chances of success for drug approval and safety.
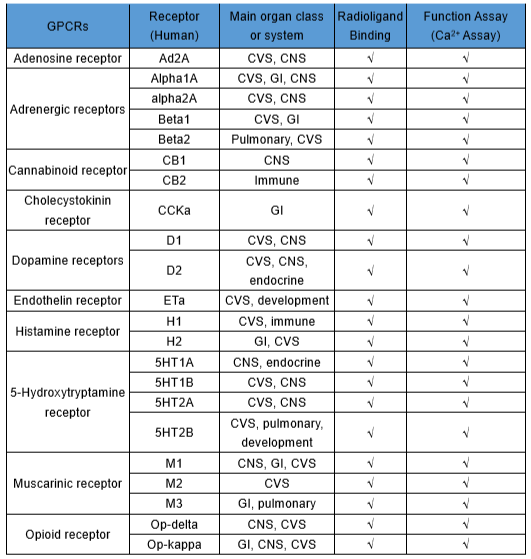
WuXi AppTec offers a in vitro mini-Safety Panel Service to be used for early pharmacological profiling of off-target and adverse effects of lead compounds or PCCs in drug development stage. Data are also useful for drug candidate SAR design. Assay targets in the panel are selected to form a minimal panel, recommended by scientists from four major pharmaceutical companies (AZ, GSK, Novartis, Pfizer)1, to provide broad and diverse early in vitro safety assessments of testing compounds. Compounds can be screened by radioligand binding, biochemical and/or functional assays.