网站维护
系统内容更新/升级中

Toxicity is the major cause of drug candidate failure in preclinical and clinical development stage, and also the major reason for the withdrawal of approved drugs from the market. While toxicity test has been traditionally completed in the preclinical phase, in vitro toxicity studies in the early drug discovery stages could significantly reduce failures at a later stage and prevent economic loss.
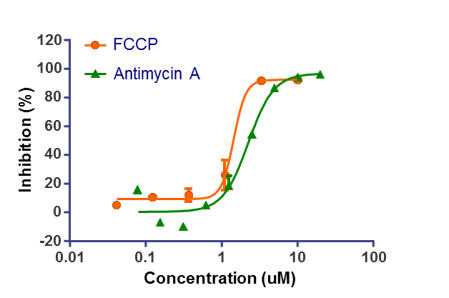
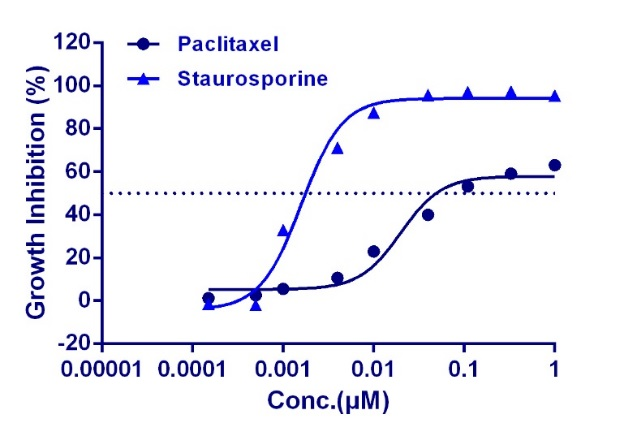
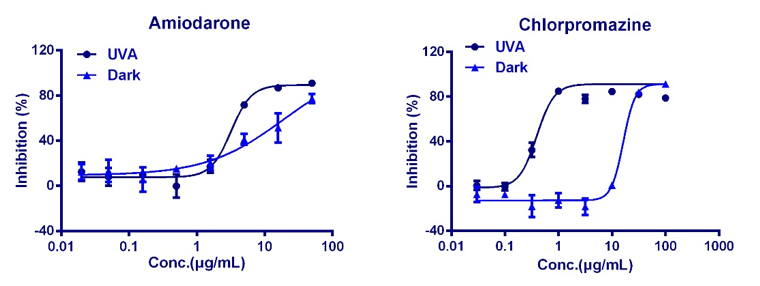
WuXi AppTec offers a panel of in vitro toxicity assays designed to identify compound toxicity in the early drug discovery stage. By utilizing cutting-edge technologies including conventional and automated patch clamp and high content screening (HCS), we provide the high-quality data quickly and cost-effectively. Applying these assays to your lead ID and optimization strategy helps to make a more thorough analysis of the severity and specificity of toxicity, then bto guide candidate compounds through the planning and execution of downstream in vivo toxicity and efficacy tests.
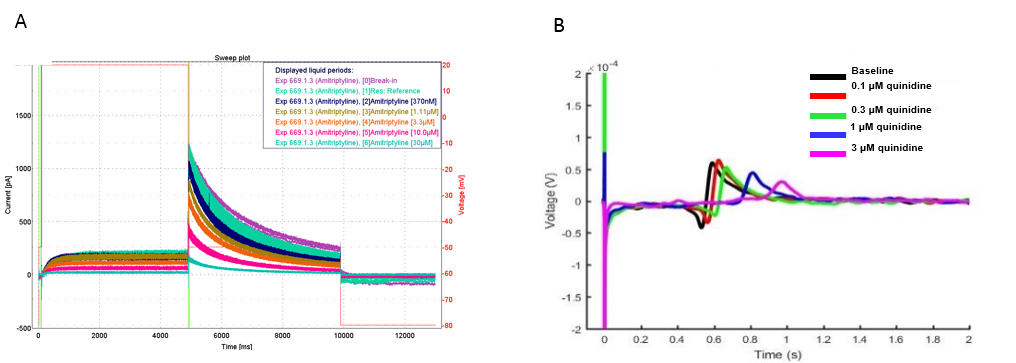
Human stem cell derived cardiomyocytes cardiotoxicity testing with MEA