网站维护
系统内容更新/升级中

We provide regulatory-driven analytical services for drug development including monitoring and quality control for APIs, intermediates, raw materials and finished products. Our services encompass method development and validation, characterization of reference materials, stability testing, routine sample analysis, GLP batch release testing and other special studies. Our experienced scientists readily make efficient and productive solutions tailored to your projects . Our diversified and best-in-class analytical instruments enable us to provide optimized services for your special needs.
In supporting CMC programs, pre-formulation, formulation and product release, our laboratories are equipped with advanced and qualified instruments; our scientists participating in these activities are fully trained; all written procedures and SOPs (standard operating procedure) are strictly followed so that data integrity is maintained with complete and accurate results and traceable records.
With a record of multiple successful on-site inspections for a number of domestic and international projects, we are willing to extend our services to support the development of your products.
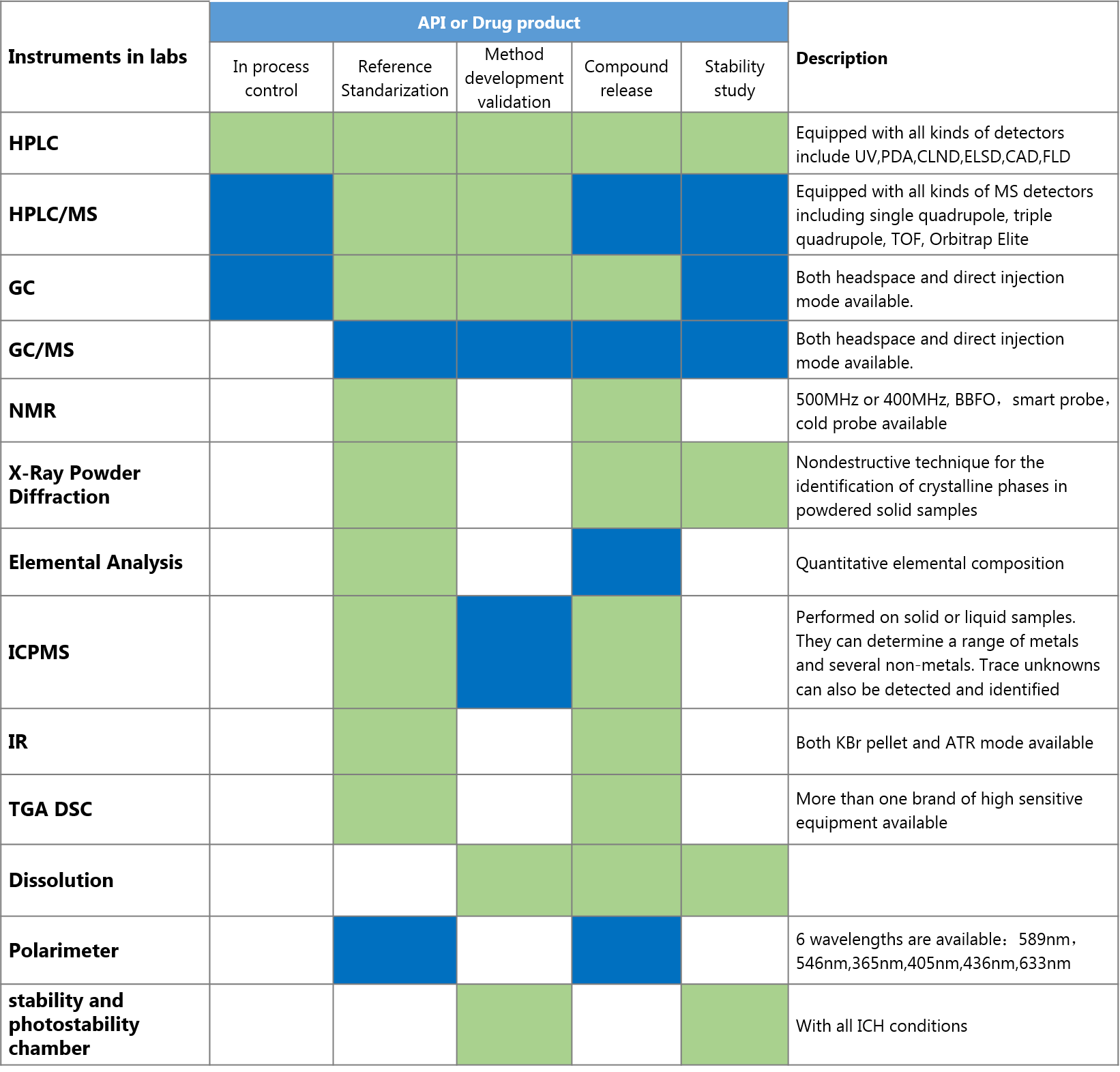
Green: Often Used Blue: Sometimes used