网站维护
系统内容更新/升级中

2020/05/20
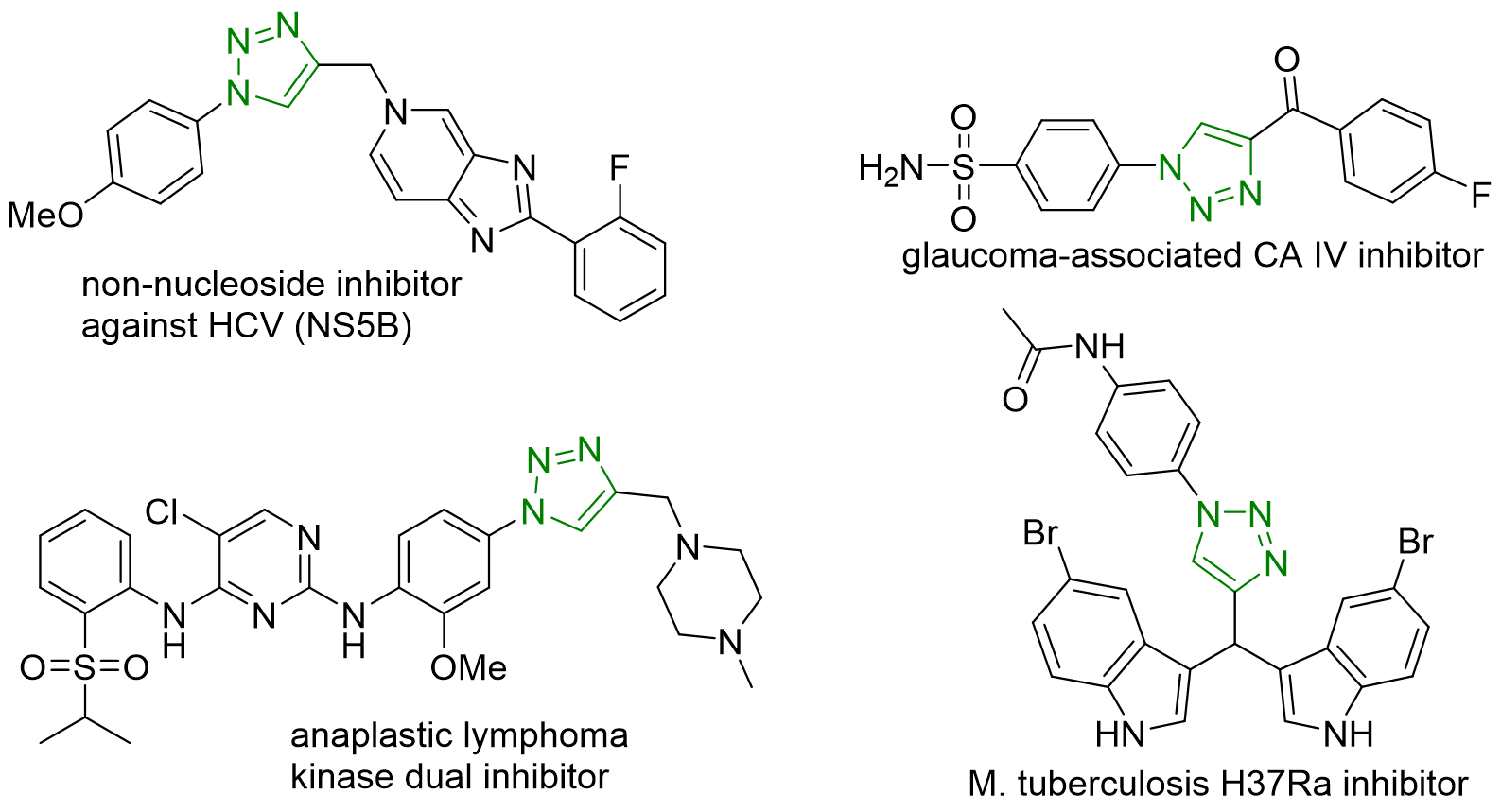
The classic click reaction is the copper-catalysed reaction of an azide with an alkyne to form a 5-membered heteroatom ring: a Cu(I)-catalysed azide-alkyne cycloaddition (CuAAC), and the corresponding product 1,2,3-triazoles are important scaffolds in natural product and medicinal agents (Scheme 1). Numerous triazole-containing molecules such as tazobactam, cefatrizine, and carboxyamidotriazole are protein inhibitors with significant biochemical activities.
Considering the importance of DNA-encoded chemical libraries (DECLs) synthesis with 1,2,3-triazoles scaffolds, Dr. Xuanjia Peng (WuXi AppTec) has recently reported a DNA-compatible copper-mediated efficient synthesis of 1,2,3-triazoles via a one-pot reaction of aryl borates with TMS-N3 followed by a click cycloaddition reaction on Organic Letters. This novel DNA-compatible reaction proceeded well with excellent functional group tolerance under mild reaction conditions, improving the application of 1,2,3-triazoles into DNA-encoded chemical libraries (DECLs) production.
Our Initial investigation focused on the examination of the one-pot reaction with DNA-conjugated phenylacetylene 1a with 5-pyrimidylboronic ester 2a. Using TMS-N3 as the azide source, we tested the one-pot reaction in borate buffer (pH 9.5) (Figure 2). We tried a variety of copper sources, and the reaction with the Cu(II)-β-cyclodextrin complex (Entry 6) gave the desired product 3a in 99% conversion.
With the optimized reaction conditions in hand, we first investigated the substrate scope of the aryl boronic acids or esters 2 (Scheme 3). The phenylboronic acids bearing phenyl and alkyl groups afforded the desired products in moderate conversions. Substrates with fluorine-containing functional groups were transformed into the desired products in good yields. Substrates with electron withdrawing groups such as ketone, acid, ester, amide, nitro, sulfonamide and cyano. Reactions were extended to various O-, S-, and N-containing heteroaromatic boronic acids, such as benzofuran, benzothiophene, pyridine, indole, and quinoline, and good yields of corresponding products were obtained. Vinyl boronic acids proceeded smoothly in moderate conversions.


In order to demonstrate the synthetic utility of this novel protocol, we tested the reaction on a tethered substrate 5 (Scheme 4), and the intramolecular macrocyclisation was successfully achieved by this transformation with useful levels of efficiency (6, 22%)
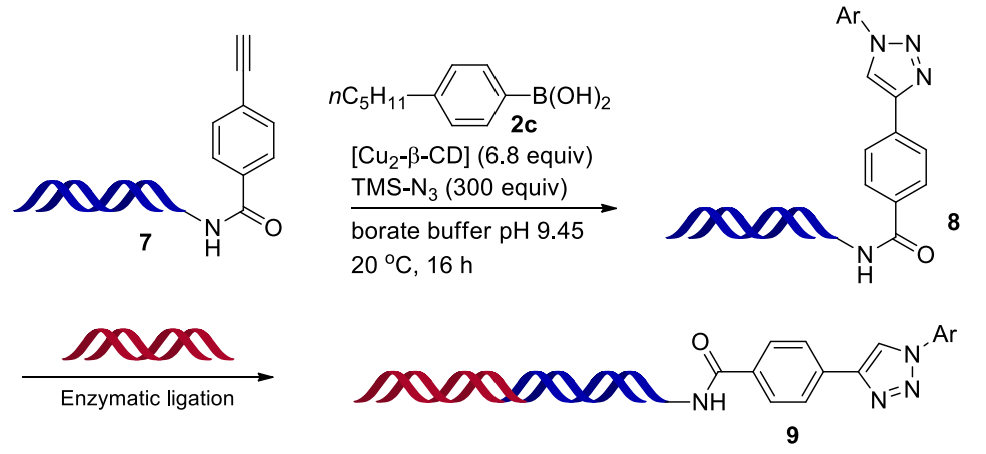
We also prepared the substrate 7 with an elongated 39-bp dsDNA tag. The copper-mediated one-pot 1,3-dipolar cycloaddition reaction between 7 and aryl borate 2c gave the desired product 8 in good conversion. Enzymatic ligation of compound 8 with another dsDNA tag gave the longer DNA product 9 with a 50-bp dsDNA tag, identified by the gel electrophoresis image (Figure 5). The damage of DNA by this chemical transformation was evaluated by qPCR analysis and next generation sequencing, showing that no DNA damage was observed.

In summary, we have developed a novel click reaction for the efficient preparation of DNA-conjugated 1,2,3-triazoles, using the binuclear macrocyclic nanocatalyst Cu(II)-β-cyclodextrin.The reaction proceeded under mild conditions with high conversions and wide functional group tolerance. Our optimized reaction protocol resulted in no significant DNA damage as judged by qPCR analysis and next generation sequencing.
Reference:
1. Qu Yi; Wen Huanan; Ge Rui; Xu Yanfen; Gao Hong; Shi Xiaodong; Wang Jiangong.; Cui Weiren; Su Wenji; Yang Hongfang; Kuai Letian; Satz Alexander L.; Peng Xuanjia. Org. Lett. 2020, DOI:10.1021/acs.orglett.0c01219.