网站维护
系统内容更新/升级中

In chapter 19 on tele-substitution of 2,3-dichloropyrazine, we learned that dichloropyrazine uses LUMO+1, not LUMO, for its nucleophilic substitution. In this chapter we will have further examples on choosing the appropriate LUMO or LUMO+1 and HOMO or HOMO-1 to correlate with electrophilic and nucleophilic reactivity trends observed in heterocyclic chemistry[1].
Shown in Figure 1 is the relative reactivities in nucleophilic substitution described in Heterocyclic Chemistry[2] for chlorodiazines and chloropyridines.
Selecting the appropriate LUMO/LUMO+1 for comparison is the key in correlating reactivity of observed. As shown in Figure 2, 4-chloropyrimidine 1 and 4-chloropyridine 6 have LUMO lobes centered on the C-Cl carbons, while have little to none in LUMO+1; as such their LUMO are used for correlation. In contrast, for 2-chloropyrimidine 2, 3-chloropyridazine 4, 2-chloropyrazine 5, and 2-chloropyridine 7, their LUMO have almost no lobe centered on the C-Cl carbons, while the LUMO+1 have significant ones; therefore their LUMO+1 are selected.
4-Chloropyridazine 3 is unique: its LUMO and LUMO+1 have energy gap of ~ 0.6 eV, and both have lobes slightly off-center on the C-Cl carbon. How do we choose the proper one to correlate?
1) Observed experimental difference: 4-Chloropyridazine 3 is less reactive than 2-chloropyrimidine 2; as such its LUMO+1, of comparable energy, is selected for correlation.
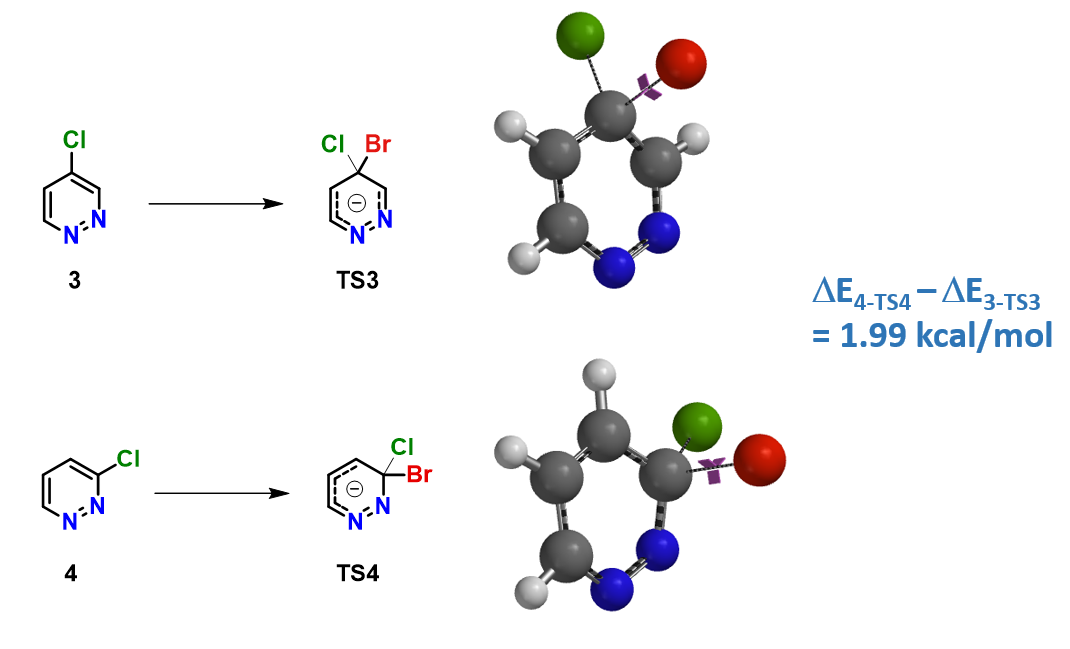
2) Activation energy difference: Activation energies for bromide substitution of 4- & 3-chloropyridazine 3 & 4 are calculated (Figure 3). ![]() E4-TS4 is found to be higher than
E4-TS4 is found to be higher than ![]() E3-TS3 by 1.99 kcal/mol, consistent with the observed difference in reactivity, and supportive of picking LUMO+1 of pyridazine 3 for reactivity correlation.
E3-TS3 by 1.99 kcal/mol, consistent with the observed difference in reactivity, and supportive of picking LUMO+1 of pyridazine 3 for reactivity correlation.
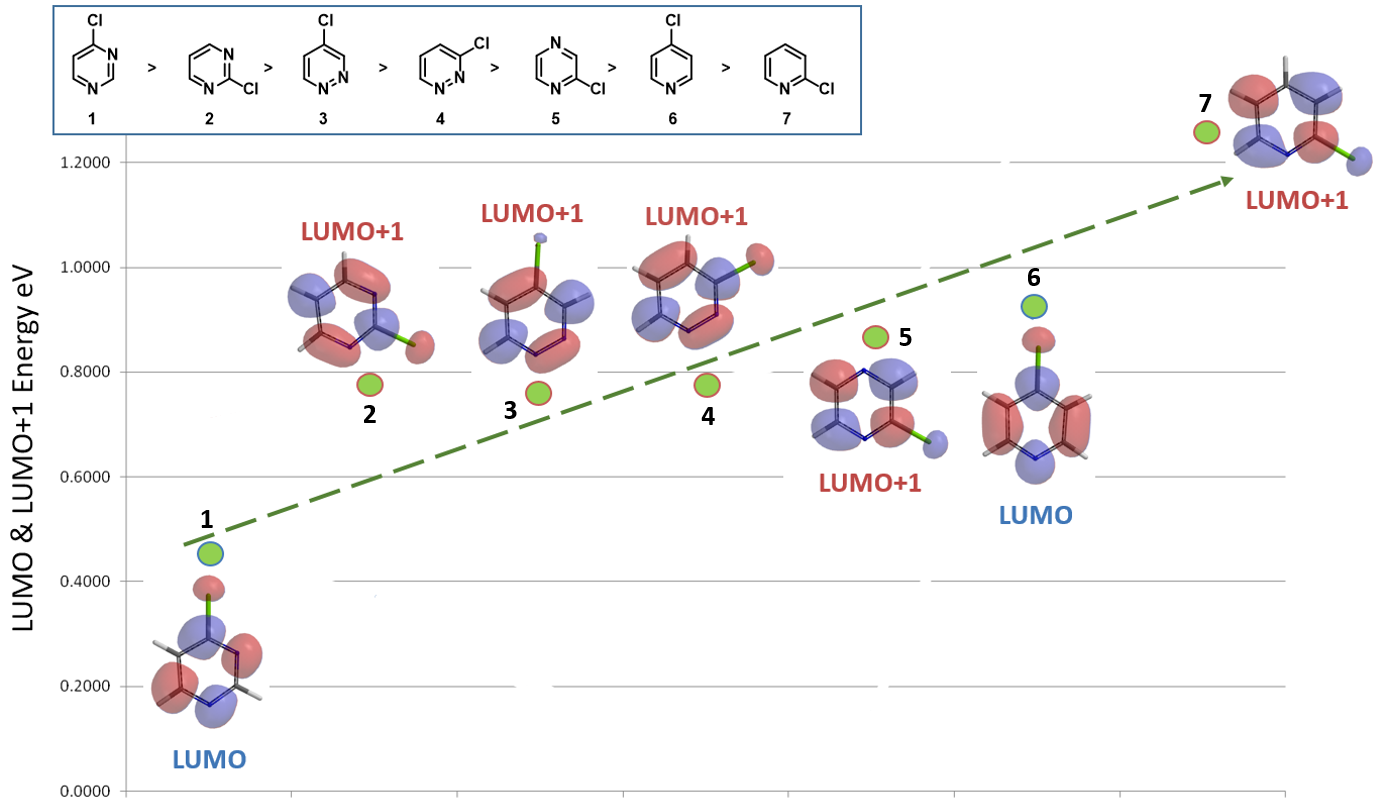
With proper LUMO/LUMO+1 selection of the above seven chlorodiazines and pyridines, a direct correlation with their reactivity becomes obvious (Figure 4). However, a new puzzle emerges: “For 4-chloropyridazine 3, with suitable LUMO lobe available for nucleophilic substitution, why does the higher energy LUMO+1 fit the trend better?”
Next, correlating reactivity of eight common 5-membered and 6-membered heterocycles with their HOMO/HOMO-1 in electrophilic substitution reaction (Figure 5).
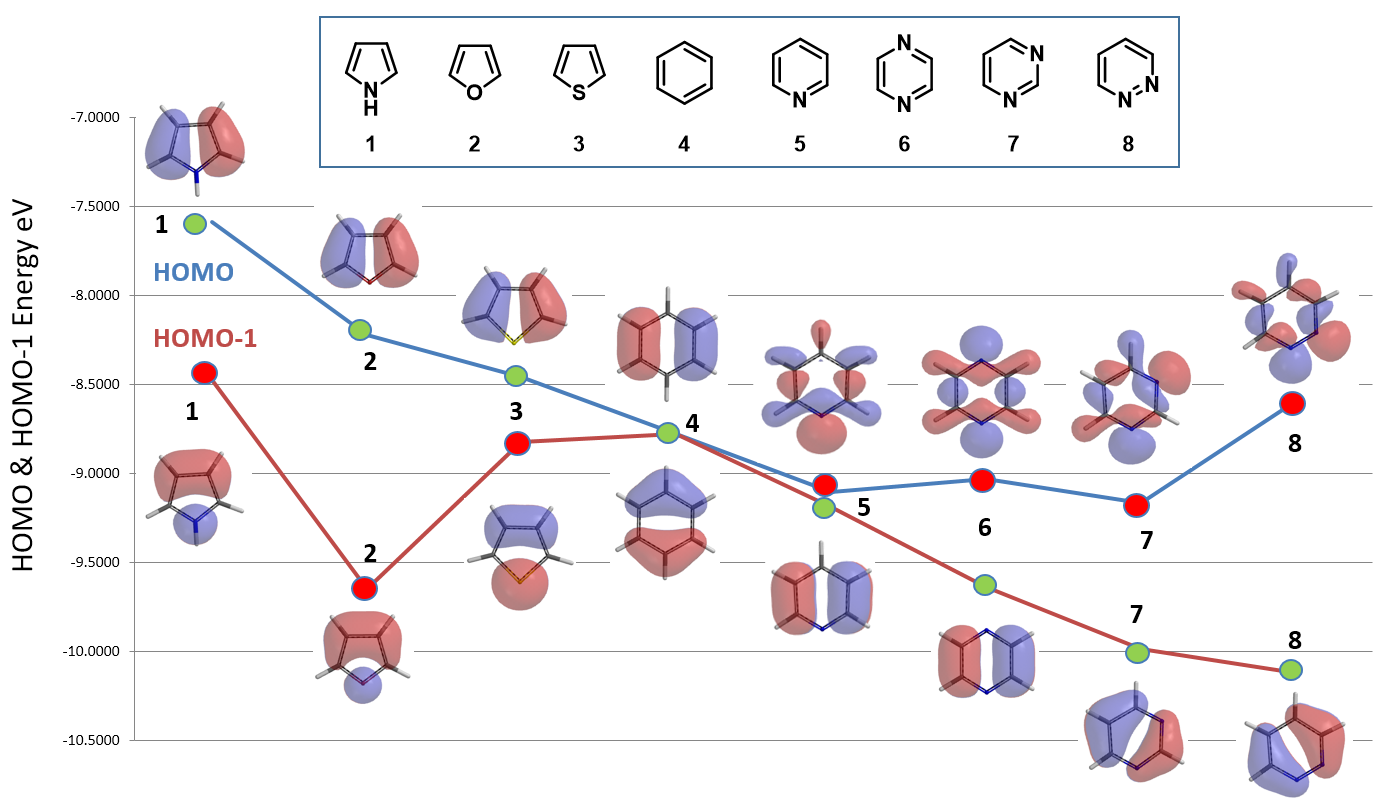
As shown in Figure 6, all the five-membered heterocyclic compounds, pyrrole 1, furan 2, and thiophene 3, have obvious HOMO lobes for electrophilic substitution. Moreover, lobes on C2 are significantly larger than on C3, consistent with higher reactivity observed at C2. Benzene 4 is C6 symmetrical: HOMO and HOMO-1 have the same energy (-8.78 eV). Each of its HOMO lobes is distributed over two carbon atoms, while the HOMO-1 ones are concentrated over one carbon atom.
For pyridine 5, pyrazine 6, pyrimidine 7, and pyridazine 8, there are significant HOMO lobes on the nitrogen atoms, while the HOMO-1 lobes are mainly distributed on the carbon atoms, with little or no distribution on the nitrogen atoms. As such their HOMO-1 shall be selected for the comparison for electrophilic reactivity occurring on the carbons.
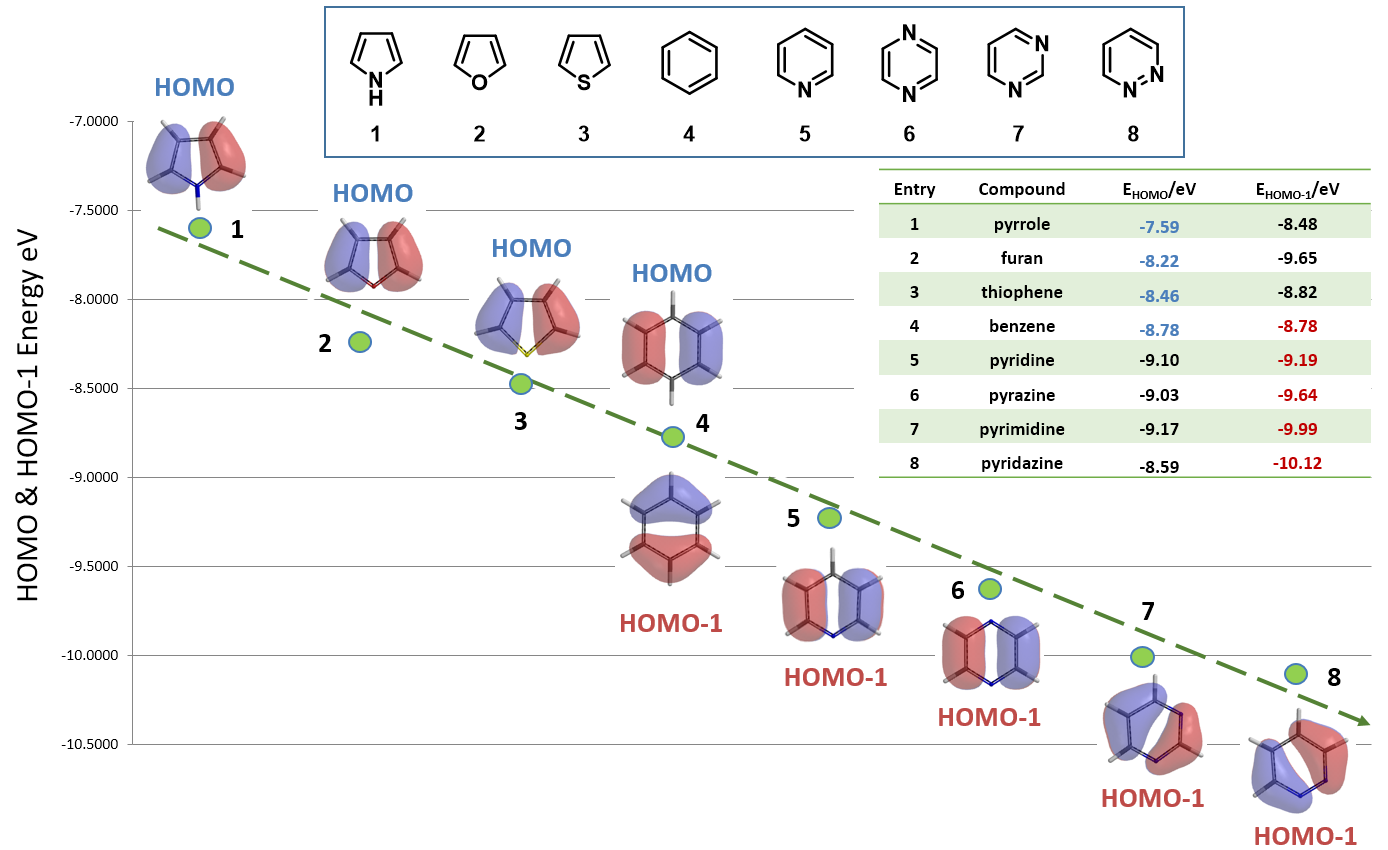
As shown in Figure 7, reactivities of the eight 5- & 6-membered heterocycles correlate well with their proper HOMO/HOMO-1 energies. The five-membered heterocyclic compounds is significantly more reactive, having higher HOMO energies than that of six-membered heterocyclic compounds. Benzene 4 stands out as unique transition from electron rich to electron deficient heterocycles.
In summary, two sets of heterocyclic compounds are used to illustrate the use of FMO analysis to correlate with their nucleophilic or electrophilic reactivities. For halogenated heterocyclic compounds, when they have LUMO lobes centered on C-X carbons, their LUMO shall be selected; when there is little/no LUMO lobes on C-X carbons, LUMO+1 shall be considered; when their LUMO and LUMO+1 lobes are comparable, we’ll need to compare calculated activation energies. Similar criteria apply for HOMO/HOMO-1 analyses.
Pyridines, with their HOMO lobes centered on the aromatic nitrogen, could be oxidized with peracid (such as peracetic acid, m-CPBA, etc.) to the corresponding N-oxides. For oxidation of electron-deficient pyridines, Caron et al.[3] reported that oxidation with urea-hydrogen peroxide complex (UHP) and trifluoroacetic anhydride (TFAA) is more efficient. What could be the reasons?
References:
[1] L.G. Zhuo, W. Liao, Z.X. Yu, Asian J. Org. Chem. 2012, 1, 336.
[2] J.A. Joule & K. Mills. Heterocyclic Chemistry 5th Ed. Chichester, West Sussex, UK: Blackwell Publishing Ltd., 2010; p 256.
[3] S. Caron, N.M. Do, J.E. Sieser, Tetrahedron Lett. 2009, 41, 2299.
This article is written and edited by Zhengquan Zhou, Dong Pan, Liting Dong, Tommy Lai, Yongsheng Chen, John S. Wai