网站维护
系统内容更新/升级中

2020/05/14
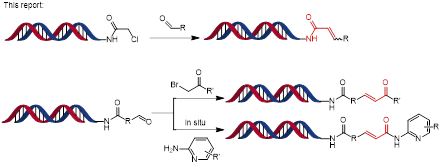
DNA-encoded chemical library (DECL) technology allows the rapid construction of large chemical libraries of typically millions to billions of compounds. New DNA-compatible reactions are desired to increase library diversity and accessible chemical space. At present, α,β-unsaturated carbonyls, recognised as potentially valuable drug motifs, have not been well investigated in DECL synthesis.
Recently, Dr. Xuanjia Peng (WuXi AppTec DEL) has reported a novel and robust DNA-compatible, PPh2CH3 mediated Wittig olefination reaction to synthesis α,β-unsaturated amides on Organic Letters (Scheme 1). This novel DNA-compatible reaction proceeded well with excellent functional group tolerance under mild reaction conditions.
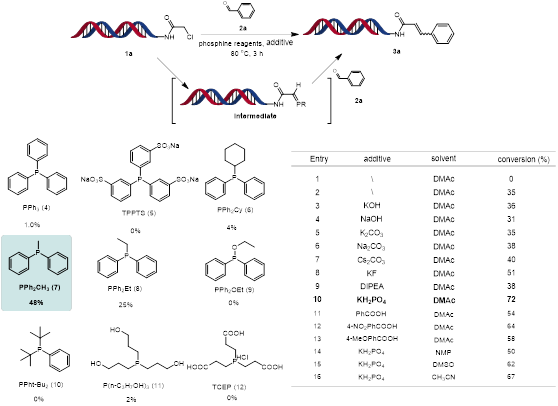
The reaction condition optimisation for this DNA-compatible Wittig olefination was performed (Scheme 2). DNA-conjugated α-chloride amide 1a was reacted with benzaldehyde 2a in the presence of Na2CO3 at 80°C for 3 h in a PCR thermos cycle instrument. A series of phosphine reagents were examined and PPh2CH3 gave the highest yield of 3a (48%). A range of acidic and basic additives and reaction solvents were investigated to improve the conversion rate. The yield of 3a improved significantly in the presence of KH2PO4. Additionally, the solvents DMAc was found to be the best solvent in this Wittig olefination reaction.
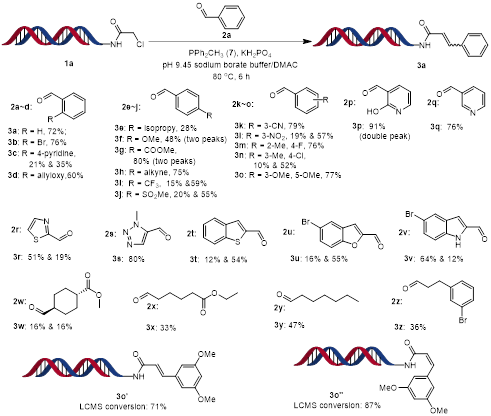
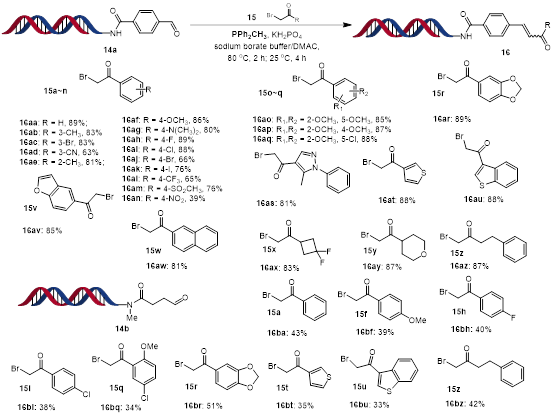
Initially, we focused on the investigation of scope of aldehyde under the optimised reaction conditions (Scheme 3). DNA-conjugated α-chloroamide 1a was reacted with different aldehydes to give α,β-unsaturated amides 3 in moderate to high yields. In most cases, the reaction gave both E and Z isomers. Aliphatic, aryl, and heteroaryl aldehydes performed well. Functional groups including aryl halides, ester, alkyne and alkene were all tolerated. Electron-deficient aryl aldehydes worked better than electron-rich aryl aldehydes. Then, DNA-conjugated aldehyde 14a was reacted with a wide range of α-halogenated carbonyl compounds (Scheme 4). Aliphatic and heteroaryl α-bromo ketones gave excellent conversions. Aryl α-bromo ketones containing electron-donating groups gave excellent conversions, however aryl α-bromo ketones containing an electron-withdrawing groups gave poor to moderate conversions.
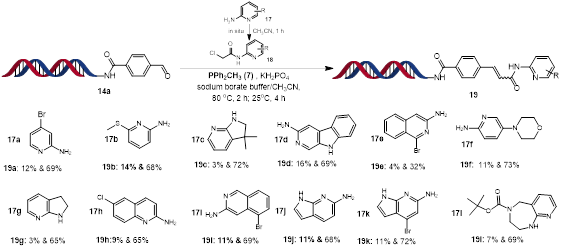
Following previous substrate scope investigation, we also performed in situ Wittig olefination reactions for DECL production (Scheme 5). We prepared a variety of α-chloroamides 18 from 2-aminopyridine analogues 17 in situ, and then reacted the resulting crude intermediates with a DNA-conjugated aldehyde 14a to give the desired Wittig olefination final products. Most substrates gave moderate conversions to the final desired product. Both primary and secondary amines could be used in this protocol. Both trans-isomer and cis-isomer products were observed.
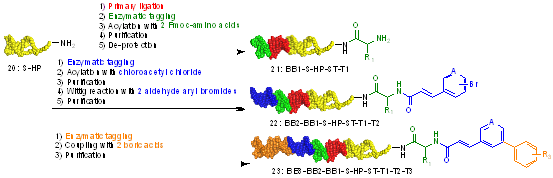
The utility of this DNA-compatible Wittig olefination was investigated, and a pilot library with three synthesis cycles was performed (Scheme 6). The library was produced in a “split and pool” strategy; eight desired products were observed after the third cycle of chemistry. Potential DNA damage following Wittig olefination was assessed with qPCR and next generation sequencing (NGS), and no significant damage were observed.
In summary, we have developed a DNA-compatible Wittig olefination reaction protocol for the efficient synthesis of DNA-conjugated α,β-unsaturated amides. A three-cycle pilot library was prepared using this DNA-compatible Wittig reaction under optimised reaction conditions. This novel strategy to generate α,β-unsaturated carbonyl compounds might be particularly useful for the design of DNA-encoded libraries capable of covalently interacting with protein targets.
Reference:
[1] An, Y. L.; Li K.; Shen, Y. F.; Hong, Z.; Chen, L.; Hu, Y.; Zhou, L.; Wang, D.; Shi, X.; Liu, S.; Su, W.; Cui, W.; Kuai, L.; Yang, H.; Peng, X. Org. Lett., 2020, DOI: 10.1021/acs.orglett.0c01215.